

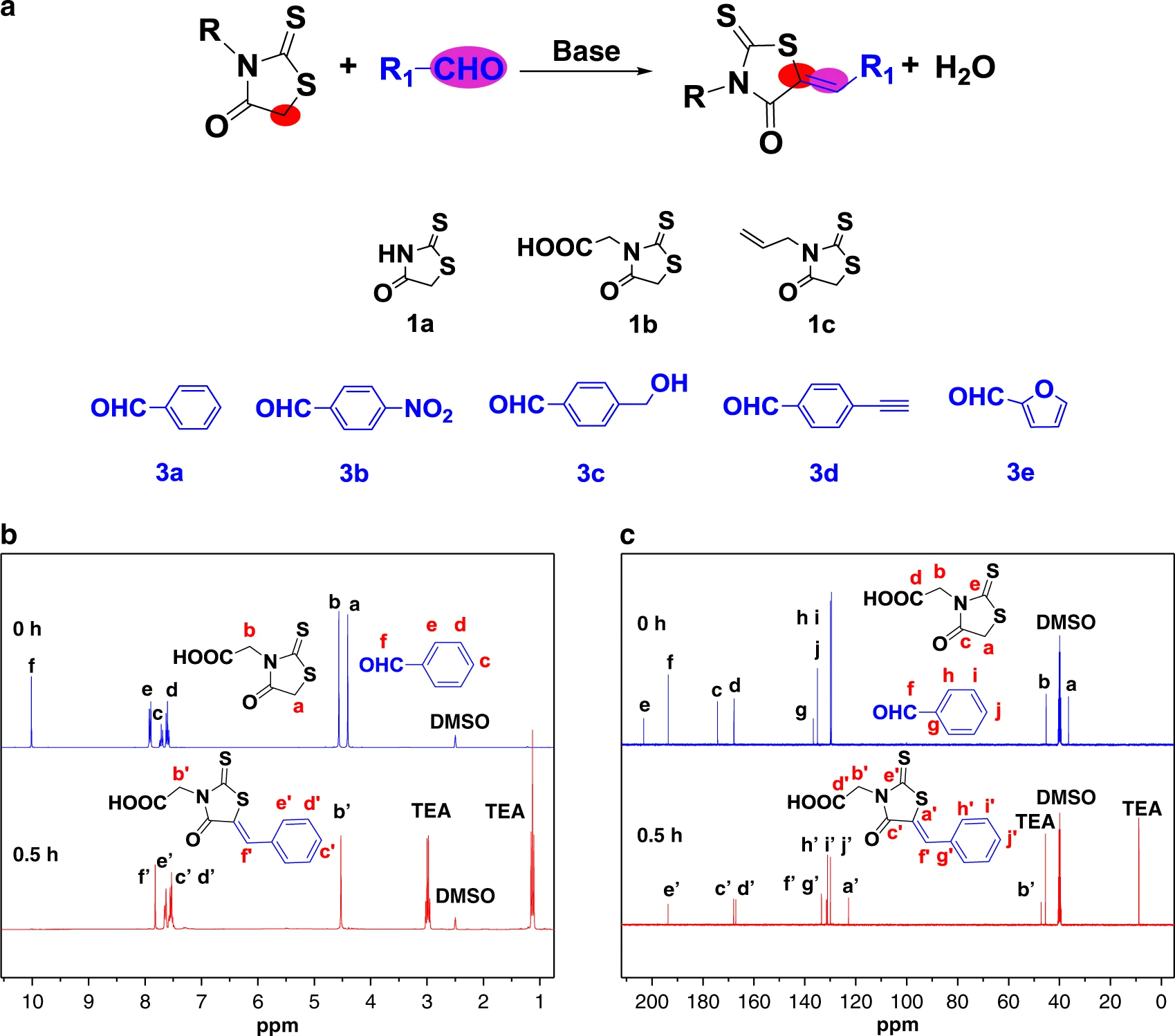
Cyclic polymers have a number of unique physical properties compared with those of their linear counterparts. However, the methods for the synthesis of cyclic polymers are very limited, and some multicyclic polymers are still not accessible now. Here, we found that the five˗membered cyclic structure and electron withdrawing groups make methylene in rhodanine highly active to aldehyde via highly efficient Knoevenagel reaction. Also, rhodanine can act as an initiator for anionic ring-opening polymerization of thiirane to produce cyclic polythioethers. Therefore, rhodanine can serve as both an initiator for ring-opening polymerization and a monomer in Knoevenagel polymerization. Via rhodanine-based Knoevenagel reaction, we can easily incorporate rhodanine moieties in the backbone, side chain, branched chain, etc, and correspondingly could produce cyclic structures in the backbone, side chain, branched chain, etc, via rhodanine˗based anionic ring-opening polymerization. This rhodanine chemistry would provide easy access to a wide variety of complex multicyclic polymers.
Autophagosome-cancer vaccines can promote cross-presentation of multiple tumour antigens and induce cross-reactive T cell responses. However, to date, there is no effective method for obtaining a highly immunogenic autophagosomal cancer vaccine because autophagosomes, once formed, quickly fuse with lysosomes and cannot easily escape from cells. Here, we report a functional Ti2NX nanodot that caps the autophagosome membrane lipid phosphatidylinositol-4-phosphate, blocking the fusion of autophagosomes with lysosomes and producing stable nanodot-coated autophagosomes in tumours. The formed nanodot-coated autophagosomes can escape from cancer cells to lymph nodes, where they activate tumour-specific T cells. We show that our approach reduces tumour burden and provide long-term immune surveillance protection for cured mice. This work provides a method for the direct formation of personalized autophagosome-based cancer vaccines in vivo, offering a promising strategy for tumour treatment.
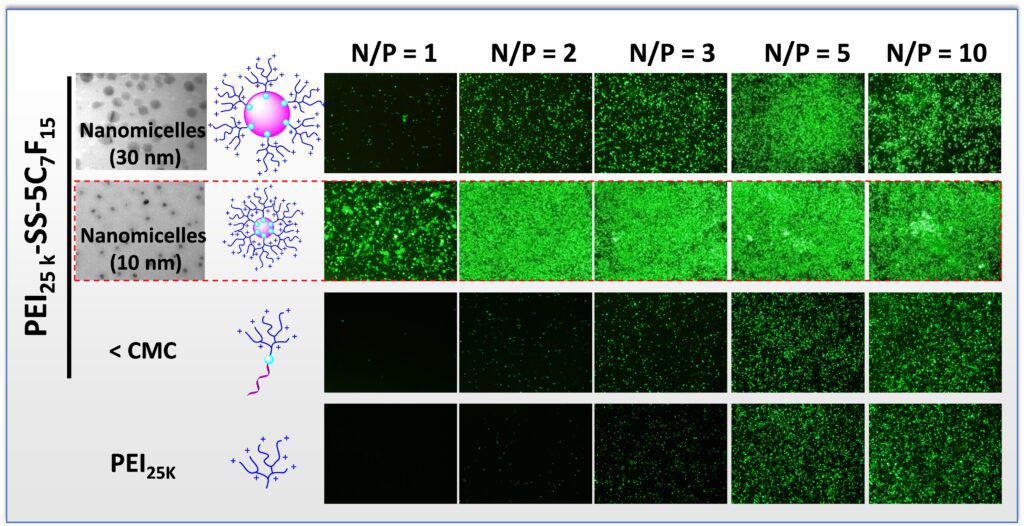
During the last two decades, cationic polymers have become one of the most promising synthetic vectors for gene transfection. However, the weak interactions formed between DNA and cationic polymers result in low transfection efficacy. Furthermore, the polyplexes formed between cationic polymers and DNA generally exhibit poor stability and toxicity because of the large excess of cationic polymer typically required for complete DNA condensation. Herein, we report the preparation of a novel class of bioreducible cationic nanomicelles by the use of disulfide bonds to connect the cationic shell to the fluorocarbon core. These bioreducible nanomicelles form strong interactions with DNA and completely condense DNA at an N/P ratio of 1. The resulting nanomicelle/DNA polyplexes exhibited high biocompatibility and performed very effectively as a gene-delivery system.
Rhodanine-based Knoevenagel reaction and ring-opening polymerization for efficiently constructing multicyclic polymers
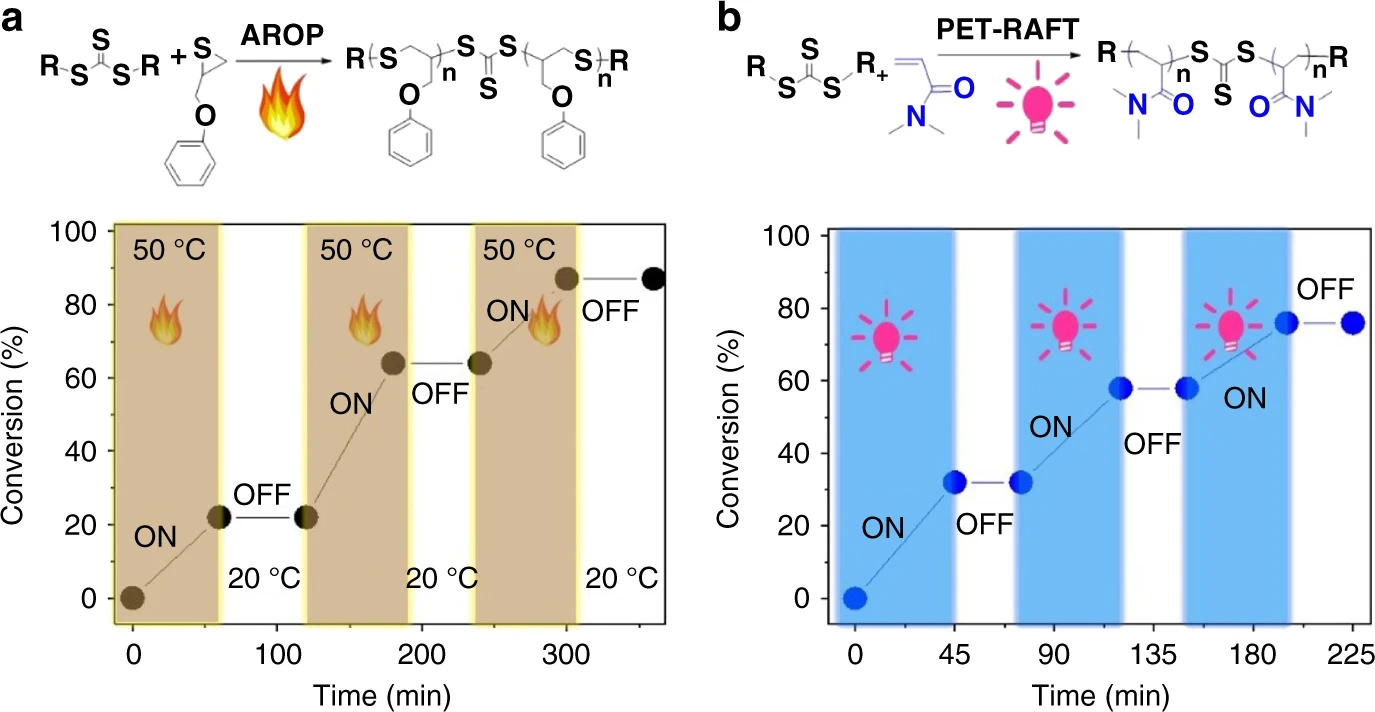
Synthesis of polymers with on-demand sequence structures via dually switchable and interconvertible polymerizations
High DNA-Binding Affinity and Gene-Transfection Efficacy of Bioreducible Cationic Nanomicelles with a Fluorinated Core
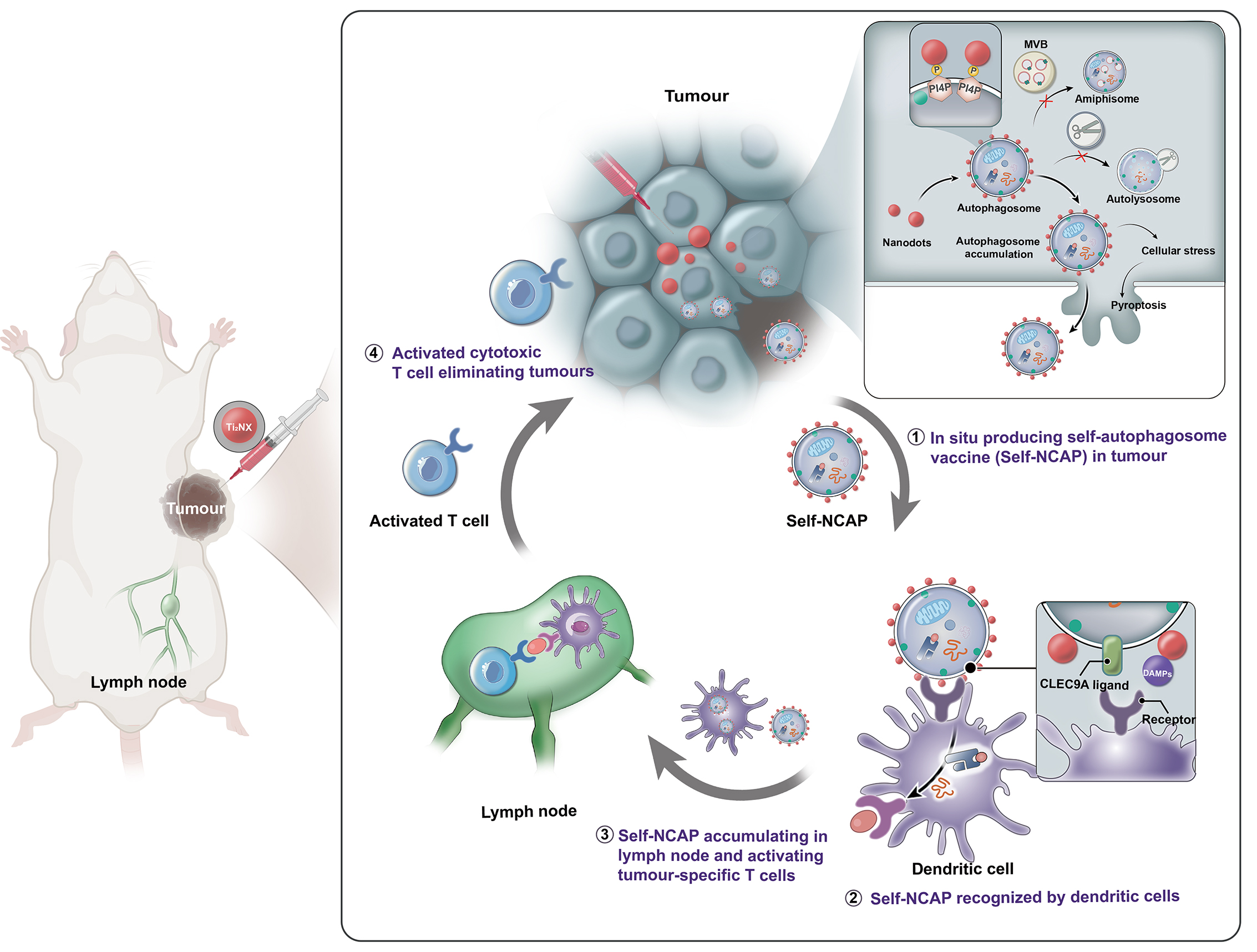
In situ autophagosome vaccine
The synthesis of polymers with on-demand sequence structures is very important not only for academic researchers but also for industry. However, despite the existing polymerization techniques, it is still difficult to achieve copolymer chains with on-demand sequence structures. Here we report a dually switchable and controlled interconvertible polymerization system; in this system, two distinct orthogonal polymerizations can be selectively switched ON/OFF independent of each other and they can be interconverted promptly and quantitatively according to external stimuli. Thus, the external stimuli can manipulate the insertion of distinct monomers into the resulting copolymer chains temporally, spatially, and orthogonally, allowing the on-demand precise arrangement of sequence structures in the resulting polymers. This dually switchable and interconvertible polymerization system provides a powerful tool for synthesizing materials that are not accessible by other polymerization methods.



